CHANLAB
PUBLICATIONS
* Denotes corresponding author
57. Krawczynska N, Wang Y, Lim K, Das Gupta A, Molino RJEJ, Lenczowski A, Abughazaleh M, Bendre SV, Fei Y, Kim H, Kockaya LI, Schane CP, Pradeep D, Rodriguez-Casiano D, Hernandez AG, Drnevich J, Chan J, Dobrucki LW, Boppart MD, Cologna SM, Ostrander J, Nelson ER. Neutrophils exposed to a cholesterol metabolite secrete extracellular vesicles that promote epithelial-mesenchymal transition and stemness in breast cancer cells. Cancer Lett. 2026, 636, 218105. article
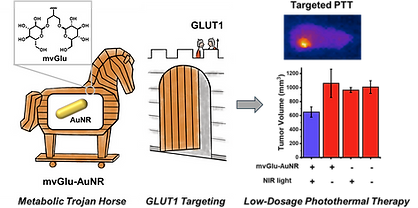
56. Jiang C‡, Bandyopadhyay S‡, Kincanon M‡, Wo A, Tedford E, East AK, Marcellus M, Murphy CJ,* Chan J.* Metabolic Trojan Horse: Multivalent Glucose Ligand Modified Near-Infrared-Absorbing Gold Nanorods for Targeted Photothermal Therapy. 2025. Submitted.
55. Forzano JA, Bandyopadhyay S, Dirak M, Mallojjala SC, Brady C, Thomas B, Hirschi J, Chan J.* Donor-PeT-Based ALDH1A1-Activated Photosensitizer for Targeted Photodynamic Therapy in NSCLC. 2025. Accepted (ACS Chem Bio).

54. Zhao Z, Lucero MY, Su S, Xu JJ, Myszka M, Chan J.* Activity-based sensing reveals elevated labile copper promotes liver aging via hepatic ALDH1A1 depletion. Nat. Comm. 2025, 16, 1794. article

53. Jiang C, Zhao Z, East AK, Bandyopadhyay S, Jiang Z, Chan J.* Logic-gated approach for targeted delivery and site-selective activation of photothermal agents in precision cancer treatment. Chem. Sci. 2025, 16, 5155-5165. article

52. Bandyopadhyay S, Forzano JA, Dirak M, Chan J.* Activatable porphyrin-based sensors, photosensitizers and combination therapeutics. JACS Au. 2025, 5, 1, 42–54. article

51. Swartchick CB, Dirak M, Wenger L, Tapia Hernandez R, Lee MC, and Chan J.* Activity-based bioluminescent logic-gate probe reveals crosstalk between the inflammatory tumor microenvironment and ALDH1A1 in cancer cells. JACS Au. 2024, ACS Editor's Choice. article
.png)
50. Alanagh HR, Fathi P, Knox HJ, Moitra P, Chan J, Pan D.* Exploring Biliverdin’s Molecular Interactions with Cu- and Fe-Based MOFs: A Unified In Vitro Study with Photoacoustic Analysis. Langmuir, 2024. article
48. Dirak M, Chan J, Kolemen S.* Optical imaging probes for selective detection of butyrylcholinesterase. J. Mater. Chem. B, 2024, Advance Article. article
47. Zhao Z, Yadav AK, Weng Y, Chan J.* Development of nitroreductase-activatable fluoroquinolone prodrugs exhibiting attenuated magnesium ion binding. Helv. Chim. Acta. 2023, 106, 10, e202300100. article Scott Denmark 70th Birthday Special Collection

46. Cheng J, Bo Y, Zhou J, Cai K, Wang Y, Feng Y, Li W, Jiang Y, Kuo S, Roy J, Anorma C, Gardner SH, Luu LM, Lau G, Chan J, Wang H,* Cheng J.* Leveraging intracellular ALDH1A1 activity for selective cancer stem-like cells labeling and targeted treatment via click reaction. PNAS 2023, 120, e2302342120. article CCIL News
45. Yadav AK, Chan J.* Activity-based bioluminescence probes for in vivo sensing applications. Curr. Opin. Chem. Biol. 2023, 74, 102310. article

44. East AK, Lee MC, Chang J, Sikander Q, Chan J.* Biomimetic approach to promote cellular uptake and enhance photoacoustic properties of tumor-seeking dyes. J. Am. Chem. Soc. 2023, 145, 7313–7322. article

43. Swartchick CB, Chan J.* Leveraging coordination chemistry to visualize metal ions via photoacoustic imaging. Curr. Opin. Chem. Biol. 2023, 74, 102312. article

42. Lee MC, Landers K, Chan J.* Activity-based photoacoustic probes for detection of disease biomarkers beyond oncology. ACS Bio Med Chem Au 2023, 3, 223–232. article

41. Lee MC, Gardner SH, Tapia Hernandez R, Chan J.* Application of AlDeSense to stratify ovarian cancer cells based on aldehyde dehydrogenase 1A1 Activity. JoVE 2023, 193, e64713. article

40. Rathnamalala CSL, Hernandez S, Lucero MY, Swartchick CB, Shaik AK, Hammer NI, East AK, Gwaltney SR, Chan J,* Scott CN.* Xanthene-based nitric oxide-responsive nanosensor for photoacoustic imaging in the SWIR window. Angew. Chem. Int. Ed. 2023, e202214855. article

39. Yadav AK, Zhao Z, Weng Y, Gardner SH, Brady CJ, Pichardo Peguero OD, Chan J.* Hydrolysis-resistant ester-based linkers for development of activity-based NIR bioluminescence probes. J. Am. Chem. Soc. 2023, 145, 1460–1469. article

38. Xu JJ, Lucero MY, Herndon NL, Lee MC, Chan J.* Comparison of a minimally invasive transthoracic approach and a surgical method for intrapleural injection of tumor cells in mice. Comp. Med. 2023, 73, 1-7. article
37. East AK, Lee MC, Smaga LP, Jiang C, Mallojjala SC, Hirschi JS, Chan J.* Synthesis of silicon-substituted hemicyanines for multimodal SWIR imaging. Org. Lett. 2022, 24, 8509–8513. article

36. Lucero MY, Gardner SH, Yadav AK, Borri A, Zhao Z, Chan J.* Activity-based photoacoustic probes reveal elevated intestinal MGL and FAAH activity in a murine model of obesity. Angew. Chem. Int. Ed. 2022, 61, e202211774. article

35. Tapia Hernandez R, Lee MC, Yadav AK, Chan J.* Repurposing cyanine photo-instability to develop NIR light-activatable nanogels for in vivo cargo delivery. J. Am. Chem. Soc. 2022, 144, 18101–18108. article UIUC Chemistry News

34. Li J, Dong Y, Wei R, Jiang G, Yao C, Lv M, Wu Y, Gardner SH, Zhang F, Lucero MY, Huang J, Chen H, Ge G, Chan J, Chen J, Sun H, Luo X, Qian X, and Yang Y. Stable, bright, and long-fluorescence-lifetime dyes for deep-near-infrared bioimaging. J. Am. Chem. Soc. 2022, 144, 14351-14362. article
33. Yadav AK, Lee MC, Lucero MY, Reinhardt, CJ, Su S, Chan J. NIR bioluminescence probe enables discovery of diet-induced modulation of tumor microenvironment via nitric oxide. ACS Cent. Sci. 2022, 8, 461-472. article

32. Zhao Z, Swartchick C, Chan J.* Targeted contrast agents and activatable probes for photoacoustic imaging of cancer. Chem. Soc. Rev. 2022, 51, 829-868. article

31. Rathnamalala C, Pino NW, Herrin B, Hooper M, Gwaltney S, Chan J,* Scott C.* Thienylpiperidine donor NIR xanthene-based dye for photoacoustic imaging. Org. Lett. 2021, 23, 7640–7644. article

30. Lucero MY, Chan J.* Photoacoustic imaging of elevated glutathione in models of lung cancer for companion diagnostic applications. Nat. Chem. 2021, 13, 1248–1256. article Behind the Paper EurekAlert GEN Illinois News Bureau Mirage News Phys.org Technology Networks

29. Gardner SH, Brady CJ, Keeton C, Yadav AK, Mallojjala SC, Lucero MY, Su S, Yu Z, Hirschi JS, Mirica LM, Chan J.* A general approach to convert hemicyanine dyes into highly optimized photoacoustic scaffolds for analyte sensing. Angew. Chem. Int. Ed. 2021, 60, 18860–18866. article

28. Lucero MY, Tang Y, Zhang CJ, Su S, Forzano JA, Garcia V, Huang X, Moreno D, Chan J.* Activity-based photoacoustic probe for biopsy-free assessment of copper in murine models of Wilson’s disease and liver metastasis. PNAS 2021, 118, e2106943118. article Beckman news

27. Lucero MY, East AK, Reinhardt CJ, Sedgwick AC, Su S, Lee MC, Chan J.* Development of NIR-II photoacoustic probes tailored for deep-tissue sensing of nitric oxide. J. Am. Chem. Soc. 2021, 143, 7196–7202. article

26. Tapia Hernandez R, Forzano JA, Lucero MY, Anorma C, Chan J.* Acoustogenic probes: A demonstration to introduce the photoacoustic effect via analyte sensing. J. Chem. Educ. 2021, 98, 2618–2624. article

25. East AK, Lucero MY, Chan J.* New directions in activity-based sensing for in vivo near-infrared imaging. Chem. Sci. 2021, 12, 3393-3405. article

24. Gardner SH, Reinhardt CJ, Chan J.* Advances in activity-based sensing probes for isoform-selective imaging of enzymatic activity. Angew. Chem. Int. Ed., 2021, 60, 5000–5009. article

23. Yadav AK, Hernandez S, Su S, Chan J.* Acoustic-based chemical tools for profiling the tumor microenvironment. Curr. Opin. Chem. Biol. 2020, 57, 114-121. article

22. Yu ZH, Reinhardt CJ, Hin-Fung T, Chan J, Au-Yeung HY. Activity-based ascorbate sensing using a copper-mediated oxidative bond cleavage. Chem. Eur. J. 2020, 26, 8794-8800. article
21. Reinhardt CJ, Xu R, Chan J.* Nitric oxide imaging in cancer enabled by steric relaxation of a photoacoustic probe platform. Chem. Sci. 2020, 11, 1587-1592. article

20. Smaga LP, Pino NW, Ibarra GE, Krishnamurthy V, Chan J.* A photoactivatable formaldehyde donor with fluorescence monitoring reveals threshold to arrest cell migration. J. Am. Chem. Soc. 2020, 142, 680-684. article

19. Yadav AK, Reinhardt CJ, Arango AS, Huff HC, Dong L, Malkowski MG, Das A, Tajkhorshid E, Chan J.* An activity-based sensing approach for the detection of cyclooxygenase-2 in live cells. Angew. Chem. Int. Ed. 2020, 59, 3307-3314. article Beckman News NSF Research News

18. Hedhli J, Kim MW, Knox HJ, Cole JA, Huynh T, Schuelke M, Dobrucki IT, Kalinowski L, Chan J, Sinusas AJ, Insana MF, Dobrucki LW. Imaging the landmarks of vascular recovery. Theranostics 2020, 10, 1733-1745. article Inside Front Cover Illinois News Bureau
17. Bearrood TE, Aguirre-Figueroa G, Chan J.* Rational design of a red fluorescent sensor for ALDH1A1 displaying enhanced cellular uptake and reactivity. Bioconjug. Chem. 2020, 31, 224-228. article Molecular Imaging Special Issue

16. Zhou EY, Knox HJ, Liu C, Zhao W, Chan J.* A conformationally-restricted aza-BODIPY platform for stimulus-responsive probes with enhanced photoacoustic properties. J. Am. Chem. Soc. 2019, 141, 17601-17609. article JACS Spotlights

15. Chen M, Knox HJ, Liu W, Tang Y, Chan J,* Yao J.* Simultaneous photoacoustic imaging of hemodynamic and tissue hypoxia. Opt. Lett. 2019, 44, 3373-3776. article OSA Editor's Pick
.png)
14. Fathi P, Knox HJ, Sar D, Tripathi I, Misra SK, Ostadhossein F, Esch MB, Chan J,* Pan D.* Biodegradable biliverdin nanoparticles for efficient photoacoustic imaging. ACS Nano. 2019, 13, 7690-7704. article Beckman News

13. Knox HJ, Chan J.* Acoustogenic probes: A new frontier in photoacoustic imaging. Acc. Chem. Res. 2018, 51, 2897-2905. article Activity-Based Sensing Special Issue

12. Zhou EY, Knox HJ, Reinhardt CJ, Partipilo G, Nilges M, Chan J.* Near-infrared photoactivatable nitric oxide donors with integrated photoacoustic monitoring. J. Am. Chem. Soc. 2018, 140, 11686-11697. article

11. Anorma C, Hedhli J, Bearrood TE, Pino NW, Gardner SH, Inaba H, Zhang P, Li Y, Feng D, Dibrell SE, Kilian KA, Dobrucki LW, Fan TM, Chan J.* Surveillance of cancer stem cell plasticity using an isoform-selective fluorescent probe for aldehyde dehydrogenase 1A1. ACS Cent. Sci. 2018, 4, 1045-1055. article ACS LiveSlides Illinois News Bureau Medical News Biocompare Photonics

10. Knox HJ, Kim TW, Zhu Z, Chan J.* Photophysical tuning of N-oxide-based probes enables ratiometric photoacoustic imaging of tumor hypoxia. ACS Chem. Biol. 2018, 13, 1838-1843. article Sensors Special Issue

9. Geng JL, Li W, Smaga LP, Sottos NR, Chan J.* Damage-responsive microcapsules for amplified photoacoustic detection of microcracks in polymers. Chem. Mater. 2018, 30, 2198-2202. article ACS Editors Choice C&EN

8. Reinhardt CJ, Zhou EY, Jorgensen MD, Partipilo G, Chan J.* A ratiometric acoustogenic probe for in vivo imaging of endogenous nitric oxide. J. Am. Chem. Soc. 2018, 140, 1011-1018. article

7. Reinhardt CJ, Chan J.* Development of photoacoustic probes for in vivo molecular imaging. Biochemistry. 2018, 57, 194–199. article Future of Biochemistry Special Issue

6. Pino NW, Davis III J, Yu Z, Chan J.* NitroxylFluor: A thiol-based fluorescent probe for live-cell imaging of nitroxyl (HNO). J. Am. Chem. Soc. 2017, 139, 18476-18479. article

5. Knox HJ, Hedhli J, Kim TW, Khalili K, Dobrucki L, Chan J.* A bioreducible N-oxide-based probe for photoacoustic imaging of hypoxia. Nat. Commun. 2017, 8, 1794-1802. article Biochemistry Viewpoint C&EN Illinois News Bureau Phys.Org AAAS My Science Medical News Technology Breaking News

4. Bearrood TE, Chan J.* Disproportionate impact of named reactions on chemical biology. Aldrichimica Acta. 2017, 50, 31-42. article

3. Zhang JJ, Smaga LP, Satyavolu NSR, Chan J,* Lu Y.* DNA aptamer-based activatable probes for photoacoustic imaging in living mice. J. Am. Chem. Soc. 2017, 139, 17225–17228. article JACS Spotlights

2. Li H, Zhang P, Smaga LP, Hoffman RA, Chan J.* Photoacoustic probes for ratiometric imaging of copper(II). J. Am. Chem. Soc. 2015, 137, 15628-15631. article

Book Chapters
3. Yadav AK, Tapia Hernandez R, Chan J.* A general strategy to optimize the performance of aza-BODIPY-based probes for enhanced photoacoustic properties. Methods in Enzymology, 2021, 657, 415-441. book chapter
2. Lucero MY, East AK, Chan J.* Near-infrared II photoacoustic probes for nitric oxide sensing. Methods in Enzymology, 2021, 657, 157-180. book chapter
1. Zhou EY, Knox HJ, Reinhardt CJ, Partipilo G, Chan J.* Near-infrared photoactivatable nitric oxide donors with photoacoustic readout. Methods in Enzymology, 2020, 641, 113-147. book chapter
Commentaries
1. Yadav AK, Chan J.* Bright dyes bring biology into focus. ACS Cent. Sci. 2017, 3, 920–921. article
Patents Filed at Illinois
9. Chan J, Swartchick CB. Bioluminescent probes to track stem cells in vivo. 2025, US Patent Application No.: 19/069,369.
8. Chan J, Yadav AK. Tumor site-activated hydrolysis-resistant esters for drug and imaging agent delivery. 2024, US20240158359A1. article
7. Chan J, Bandyopadhyay S. Porphyrin-based photosensitizer for the treatment of cancer. 2023, US Patent Application No.: 63/593,094 and PCT Application No.: PCT/US2024/052778.
6. Chan J, East A. Tumor-seeking glucose-dendrons for the delivery of chemotherapeutics and imaging agents to cancer cells. 2022, US Patent Application No.: 63/399,678.
5. Chan J, Tapia-Hernandez R. NIR-light activatable nanogel delivery system. 2022, US Patent Application No.: 63/399,702.
4. Chan J, Reinhardt CJ, Yadav AK. Fluorescent probe for cyclooxygenase-2. 2021, US20210330822A1. article
3. Chan J, Bearrood TE, Anorma C. Selective fluorescent probe for aldehyde dehydrogenase. 2020, US20200199092A1. article
2. Chan J, Pino NW. Thiol-based fluorescent probe for reactive species. 2020, US20200140452A1. article
1. Chan J, Knox HJ. Bioreducible N-oxide-based probes for imaging of hypoxia. 2020, US20200062784A1. article
Before Illinois
25. Xiao T, Ackerman CM, Carroll EC, Jia S, Hoagland A, Chan J, Thai B, Liu CS, Isacoff EY, Chang CJ. Copper regulates rest-activity cycles through the locus coeruleus-norepinephrine system. Nat. Chem. Biol. 2018, 14, 655–663. article
24. Ladomersky E, Khan A, Shanbhag V, Cavet JS, Chan J, Weisman GA, Petris MJ. Host and pathogen copper-transporting P-type ATPases function antagonistically during Salmonella infection. Infect. Immun. 2017, 85, e00351-17. article
23. Chun H, Sharma AK, Lee J, Chan J, Jia S, Kim B-E. The intestinal copper exporter CUA-1 is required for systemic copper homeostasis in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 1-14. article
22. Krishnamoorthy L, Cotruvo Jr JA, Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Vander Wal MN, Guan T, Smaga LP, Farhi SL, New EJ, Lutsenko S, Chang CJ. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016, 12, 586–592. article
21. Nantasanti S, Spee B, Kruitwagen HS, Chen C, Geijsen N, Oosterhoff LA, van Wolferen ME, Pelaez N, Fieten H, Wubbolts RW, Grinwis GC, Chan J, Huch M, Vries RRG, Clevers H, de Bruin A, Rothuizen J, Penning LC, Schotanus BA. Disease modelling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Reports 2015, 5, 895-907. article
20. Chan J, Tang A, Bennet AJ. Transition-state structure for the hydronium ion-promoted hydrolysis of α-D-glucopyranosyl fluoride. Can J Chem 2015, 93, 463-467. article
19. Dodani SC,* Firl A,* Chan J,* Nam CI, Aron AT, Onak CS, Ramos-Torres KM, Paek J, Webster CM, Feller MB, Chang CJ. Copper is an endogenous modulator of neural circuit spontaneous activity. PNAS 2014, 111, 16280-1625. [* = equal contribution] article
18. Hong-Hermesdorf A, Miethke M, Gallaher SD, Kropat J, Dodani SC, Chan J, Barupala D, Domaille DW, Shirasaki DI, Loo JA, Weber PK, Pett-Ridge J, Stemmler TL, Chang CJ, Merchant SS. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat. Chem. Biol. 2014, 10, 1034-1042. article
17. Chan J, Sannikova N, Tang A, Bennet J. Transition-state structure for the quintessential SN2 reaction of a carbohydrate: Reaction of α-glucopyranosyl fluoride with azide ion in water. J. Am. Chem. Soc. 2014, 136, 12225-12228. article
16. Kashyap DR, Rompca A, Gaballa A, Helmann JD, Chan J, Chang CJ, Gupta D, Dziarski R. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathog. 2014, 10, 1-17. article
15. Polishchuk EV, Concilli M, Iacobacci S, Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van IJzendoorn SCD, Chan J, Chang CJ, Amoresano A, Pane F, Pucci P, Tarallo A, Parenti G, Brunetti-pierri N, Settembre C, Ballabio A, Polishchuck RS. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell. 2014, 29, 686-700. article
14. Chan J, Chang CJ. Making light of stress. Nat. Biotechnol. 2014, 32, 337-338. article
13. Huang CP, Fofana M, Chan J, Chang CJ, Howell SB. Copper transporter 2 regulates intracellular copper and sensitivity to cisplatin. Metallomics 2014, 6, 654-661. article
12. Hao Z, Lou H, Zhu R, Zhu J, Zhang D, Zhao BS, Zeng S, Chen X, Chan J, He C, Chen PR. The multiple antibiotic resistance regulator MarR is a copper sensor in Escherichia coli. Nat. Chem. Biol. 2014, 10, 21-28. article
11. Au-Yeung HY, Chan J, Chantarojsiri T, Chang CJ. Molecular imaging of labile iron(II) pools in living cells with a turn-on fluorescent probe. J. Am. Chem. Soc. 2013, 135, 15165–15173. article
10. Chan J, Dodani SC, Chang CJ. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 2, 973–984. article
9. Macauley MS, Chan J, Zandberg W, He Y, Whitworth GA, Stubbs KA, Yuzwa SA, Bennet AJ, Varki A, Davies GJ, Vocadlo DJ. Metabolism of vertebrate amino sugars with N-glycolyl groups: Intracellular O-GlcNGc, UDP-GlcNGc, and the biochemical and structural rationale for the substrate tolerance of O-GlcNAcase. J. Biol. Chem. 2012, 287, 28882–28897. article
8. Chan J, Lewis AR, Indurugalla D, Schur M, Wakarchuk W, Bennet AJ. Transition state analysis of Vibrio cholerae sialidase-catalyzed hydrolyses of natural substrate analogues. J. Am. Chem. Soc. 2012, 134, 3748–3757. article
7. Chan J, Tang A, Bennet AJ. A stepwise solvent-promoted SNi reaction of α-D-glucopyranosyl fluoride: mechanistic implications for retaining glycosyltransferases. J. Am. Chem. Soc. 2012, 134, 1212–1220. article
6. Chan J, Watson JN, Lu A, Borgford TJ, Bennet AJ. Bacterial and viral sialidases: Contribution of the conserved active site glutamate to catalysis. Biochemistry 2012, 51, 433–441. article
5. Chan J, Bennet AJ. "Enzymology of influenza virus sialidase" in Influenza Virus Sialidase - A Drug Discovery Target, M. Von Itzstein (ed); Springer: New York, 2012, 47-66. article
4. Chan J, Sandhu G, Bennet AJ. A mechanistic study of sialic acid mutarotation: Implications for mutarotase enzymes. Org. Biomol. Chem. 2011, 9, 4818–4822. article
3. Chan J, Lu A, Bennet AJ. Turnover is rate-limited by deglycosylation for Micromonospora viridifaciens sialidase-catalyzed hydrolyses: Conformational implications for the Michaelis complex. J. Am. Chem. Soc. 2011, 133, 2989–2997. article
2. Telford JC, Yeung JHF, Xu G, Kiefel MJ, Watts AG, Hader S, Chan J, Bennet AJ, Moore MM, Taylor GL. The Aspergillus fumigatus sialidase is a 3-deoxy-d-glycero-d-galacto-2-nonulosonic acid hydrolase (KDNase): Structural and mechanistic insights. J. Biol. Chem. 2011, 286, 10783–10792. article
1. Chan J, Lewis AR, Gilbert M, Karwaski M-F, Bennet AJ. A direct NMR method for the measurement of competitive kinetic isotope effects. Nat. Chem. Biol. 2010, 6, 405–407. article
Patents Before Illinois
2. Homoallylamines as formaldehyde-responsive triggers. 2017, WO2017034927A1. article
1. Fluorescent probes for detection of copper. 2014, US20140051863A1. article

